About the Project
 |
||||||||||||||||||||||||||
Current Status of the Project
Search the Sequence
You can download the data from our FTP site. Related Links
|
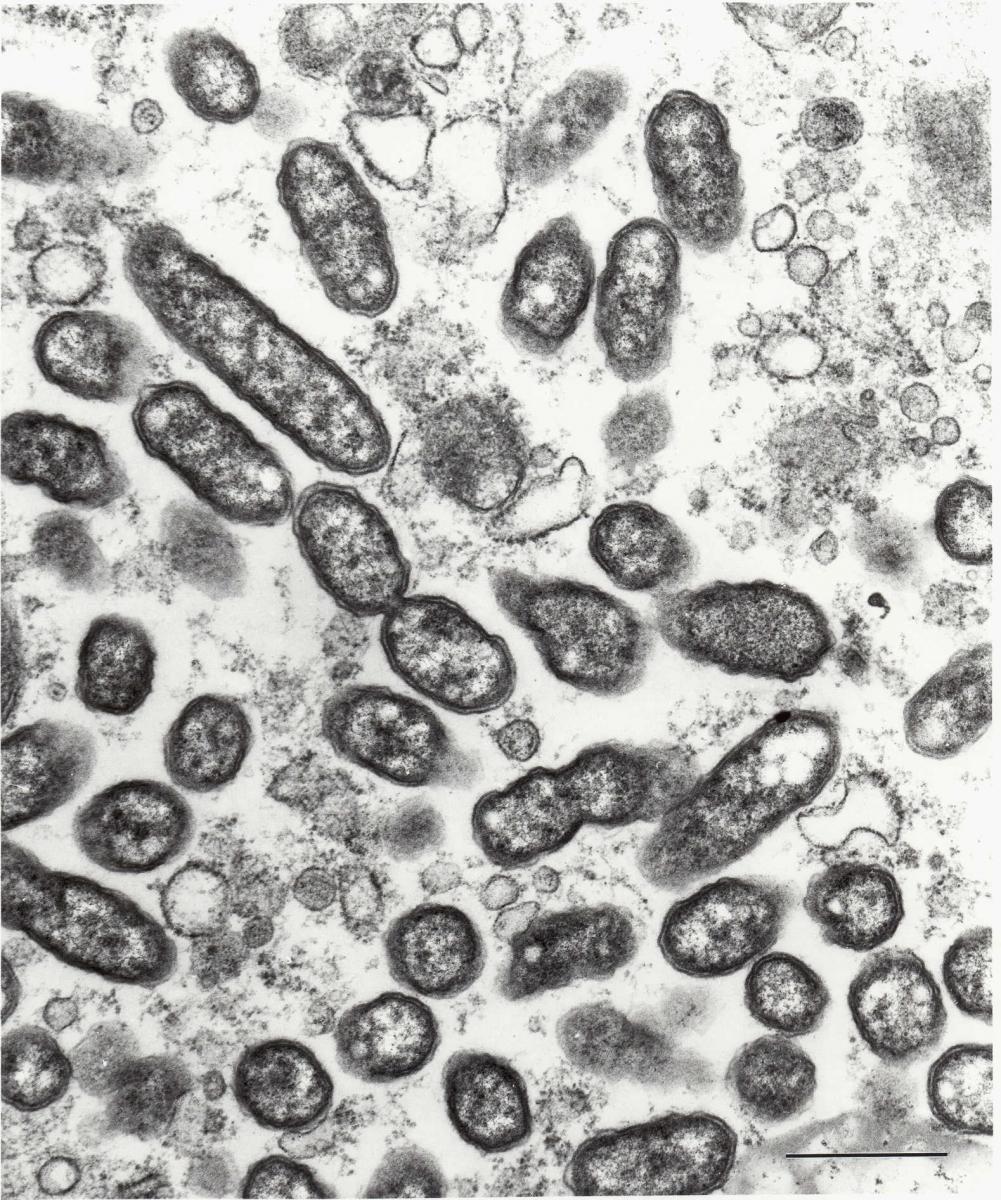
Rickettsia typhi is a small (0.3 by 1 (m), obligately intracellular bacterium. Its cell wall morphology is that of a gram-negative bacillus. Phylogenetically a member of the alpha subgroup of Proteobacteria, R. typhi is along with R. prowazekii considered to be a typhus group rickettsia. Its typhus group rickettsial characteristics include the cell wall content of abundant lipopolysaccharide containing group-specific epitopes and a major outer membrane protein designated as OmpB or specific protein antigen (SPA). OmpB is found in all the species of Richettsia, where it is encoded as a 168 kDa precursor protein that contains a 32 kDa segment that is genetically homologous to a (-autotransporter that is proposed to form a (-barrel in the cell wall and to mediate transport of OmpB to the surface of the cell wall. There it comprises the abundant S-layer in a polygonal arrangement of the mature 135 kDa proteins from which the (-autotransporter has been posttranslationally removed. OmpB of R. typhi contains conformational, species-specific epitopes. Many more metabolic studies have been performed with R. prowazekii than R. typhi. In general rickettsiae have evolved numerous characteristics that take advantage of their intracytosolic niche to acquire ATP, amino acids, cytoplasmic forms of sugars, and other metabolic products of the host cell.
Rickettsia typhi evolved in close association with its arthropod vectors, various species of fleas. In its best known zoonotic cycle, R. typhi is acquired from the blood meal taken from a bacteremic Rattus species host. The rickettsiae invade and grow within the midgut epithelial cells of the flea from which they are shed into the flea feces. The flea feces is infective for rats and human, presumably via intracutaneous inoculation by scratching the pruritic skin, inhalation of aerosolized flea feces, or introduction by the fingers into the mucous membranes such as the conjunctiva. Rats are rickettsemic without signs of illness, and rat fleas (Xenopsyble cheopis) remain infected for the rest of their lifespan, which is not affected by the infection. Intracellularly only a small proportion of R. typhi stimulate the host cell generation of short F-actin tails at one pole of the organism. Thus R. typhi do not spread effectively to other cells until their intracellular growth by binary fission results in accumulation of such large quantities of cytoplasmic bacteria that the infected host cell bursts, releasing large quantities of rickettsiae.
Rickettsia typhi are highly infectious, including by aerosol inhalation. The organisms spread hematogenously throughout the human body and infect mainly endothelial cells. The subsequent pathologic effects include meningoencephalitis, maculopapular rash, interstitial pneumonia leading to adult respiratory distress syndrome in some patients, disseminated vascular lesions, and death in approximately 1% of cases. Recovery from infection and immunity, as determined in studies of experimental infections of mice, requires cytokines (particularly gamma interferon) and CD8 T-lymphocytes, which are associated with cytotoxic T-lymphocyte activity, and is assisted by natural killer cells and antibody.